

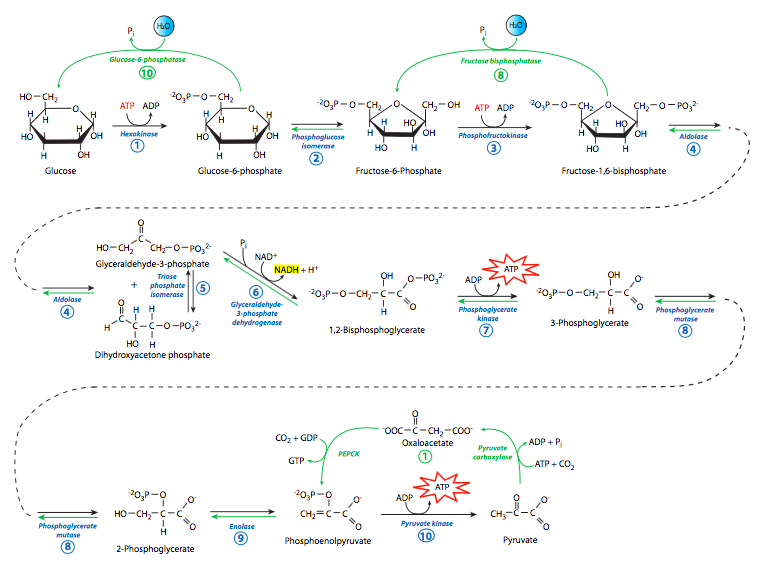
Since there is this parallel, we will explore gluconeogenesis first by starting with one of the major products of glycolysis, pyruvate. Pyruvate can be converted to oxaloacetate by pyruvate carboxylase, in a reaction requiring ATP hydrolysis. The oaxaloacetate is then converted to phosphoenolpyruvate (PEP) by PEP carboxykinase, which also uses nucleotide triphosphate hydrolysis for energy, though this time it is GTP.
Interestingly, PEP carboxykinase (PEPCK) is unregulated at the protein level. There are no known activators or inhibitors of its activity. The only regulation of PEPCK appears to be at the level of transcription: glucagon can stimulate it (as can gluocorticoids and thyroid hormone), while insulin can inhibit it. The other gluconeogenic enzymes, though, do have direct activators and inhibitors. They are allosteric modulators, binding away from, but influencing the shape and efficacy of the substrate binding site. In examining the regulation of these enzymes, one important regulator stands out because it is not a metabolite of either glycolysis or gluconeogenesis. Fructose-2,6-bisphosphate (F2,6P) is an activator of phosphofructokinase, and an inhibitor of fructose bis-phosphatase. F2,6P levels are controlled by fructose-bis-phosphatase-2 and phosphofructokinase-2, which are themselves controlled by levels of fructose-6-phosphate, as well as through a hormone-driven signaling cascade shown in the Figure on the next page.
As shown in the summary/comparison (Figure \(\PageIndex\)), from the formation of PEP to the formation of fructose-1,6-bisphosphate the enzymes used in gluconeogenesis are exactly the same enzymes used in glycolysis. This works because the free energy change in those reactions is relatively small. However, in the dephosphorylation of fructose-1,6-bisphosphate to to fructose-6-phosphate, and subsequently in the dephosphorylation of glucose-6-phosphate to glucose, there is a large free energy change that works against the gluconeogenic reactions. Thus, the enzymes that drive these reactions are different from the enzymes that drive the reverse reactions in glycolysis (i.e. hexokinase, phosphofructokinase). These two hydrolytic reactions are catalyzed by fructose bis-phosphatase and glucose-6-phosphatase, respectively. Full reversal of glycolysis in animals is limited, however, to liver and kidney, since they are the only tissues that express glucose-6-phosphatase. Other tissues use different mechanisms for generating glucose (e.g. glycogenolysis).
The glyoxylate cycle provides a mechanism for plants to convert acetyl-CoA into oxaloacetate, and therefore contribute to gluconeogenesis. This allows them to convert fatty acids and the hydrophobic amino acids leucine and isoleucine into glucose when necessary. The ability to do this comes from a plant-specific organelle called the glyoxysome, as well as some mitochondrial enzymes. The glyoxysomal part of the cycle consists of ve steps, of which the first three contribute to the conversion, while the last two steps regenerate the glyoxysomal oxaloacetate (Figure \(\PageIndex\)).
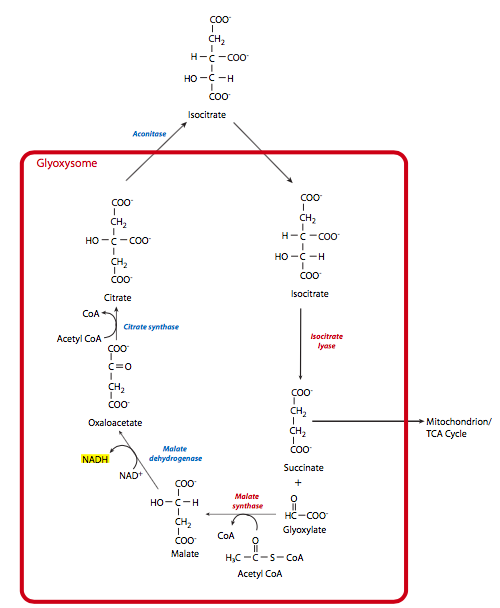
So, to summarize, the pool of oxaloacetate within the glyoxysome is used and regenerated within the glyoxysome. Acetyl-CoA is converted to succinate within the glyoxysome, but then goes to the mitochondrion for conversion to malate, and finally the cytosol for conversion to a separate pool of oxaloacetate that is then used in gluconeogenesis.
This page titled 6.4: Gluconeogenesis is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, and/or curated by E. V. Wong via source content that was edited to the style and standards of the LibreTexts platform.